How to Inject Increlex
How to Inject Increlex
How to Inject Increlex
Watch this video to find out how to prepare and inject the correct dose of Increlex
Goal of Treatment
Goal of Treatment
Goal of Treatment
Working with your child’s doctor to find the right treatment is very important in helping manage Severe Primary insulin-like growth factor-1 (IGF-1) Deficiency.
There is a window of time when your child is able to grow. Once your child enters the teen years, the potential to grow slows – and eventually stops – when the growth plates (epiphyses) close.
What to Expect
What to Expect
What to Expect With INCRELEX®
It is important to have realistic expectations of treatment with INCRELEX®, as changes to your child’s height may take time to be apparent.
GROWTH RATE WITH INCRELEX® OVER 8 YEARS3*
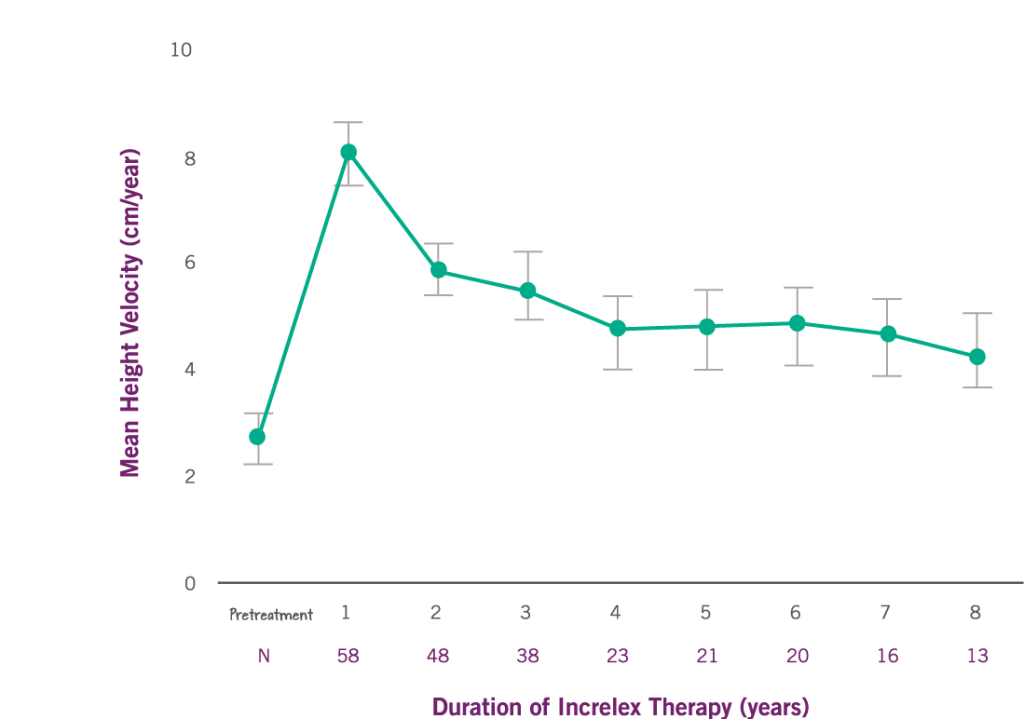
*Data comes from a clinical trial with 71 children with severe primary IGF-1 deficiency, Children aged > 2 years of age were eligible to enroll in the study. The mean age for these patients at baseline (before treatment) was: 7.8 years ± 4.5 years. The rate of growth pre-treatment (referred to as pretreatment height velocity) was available and is shown above for 58 patients. N is the number of patients evaluated for each year of therapy.
Results:
The average rate of growth (referred to as height velocity) increased by 8 cm per year in the first year from a baseline of 2.8 cm per year. The increase in height from year 1 compared to baseline was statistically significant (P < 0.0001).
The average rate of growth (referred to as height velocity) sustained by 5 cm per year in years 2 through 6 of treatment.
Patients experienced increased rate of height growth without increasing the rate of chronological bone age. Bone age is used to categorize the degree of skeletal maturation.
*Forty-nine subjects were included in an analysis of the effects of Increlex on the way your child’s bone changes in size and shape (referred as bone age). The mean ± SD change in chronological age was 4.9 ± 3.4 years and the mean ± SD change in bone age was 5.3 ± 3.4 years.
In clinical trials, INCRELEX® improved statural growth in patients diagnosed with Severe Primary IGFD
The response to INCRELEX® will vary from child to child.
You can help your doctor by regularly measuring your child’s height and monitoring their weight. Discuss with the doctor any changes to your child’s height and weight, as these will help to determine whether the dose of INCRELEX® needs to be changed.
What to Know About INCRELEX® Dose Adjustment
Regular weight monitoring and tolerability are critical for correct INCRELEX® dosing. Talk to your child’s doctor if their weight has changed so that they can determine the correct dose of INCRELEX®.


