


Please see Indication and Important Safety Information below.
On this page

Olive, a former INCRELEX patient, at age 18
What Is INCRELEX®?
INCRELEX® is a liquid that contains man-made insulin-like growth factor-1 (IGF-1).
INCRELEX® works by replacing missing IGF-1 in the body.
INCRELEX® is used to treat children who are very short for their age because their bodies do not make enough IGF-1.
This condition is called primary IGF-1 deficiency.
IGF-1 should not be used instead of growth hormone.
It is not known if INCRELEX® is safe and effective in children under 2 years of age.
How INCRELEX® Is Thought to Work
INCRELEX® replaces missing IGF-1 in the body which is a naturally occurring hormone that is essential for childhood growth.
Watch the video to find out how INCRELEX® is thought to work.
Who Should Not Receive INCRELEX®?
Your child should not receive INCRELEX® if your child:
- is allergic to mecasermin or any of the ingredients in INCRELEX®;
- has finished growing;
- has any cancerous tumors or growths;
- or has a history of cancer.
What Should I Tell My Child’s Doctor Before My Child Starts INCRELEX®?
What Are Possible Side Effects of INCRELEX®?
Low blood sugar (hypoglycemia).
INCRELEX® may lower blood sugar levels. It is important to only give your child INCRELEX® 20 minutes before or 20 minutes after a meal or snack to reduce the chances of low blood sugar. Do not give your child INCRELEX® if your child cannot eat. Signs of low blood sugar include: dizziness; tiredness; restlessness; hunger; irritability; trouble concentrating; sweating; nausea; and fast or irregular heartbeat. Severe low blood sugar may cause unconsciousness, seizures, or death. Your child should avoid participating in high risk activities (e.g., driving, exercise, etc.) within 2 to 3 hours after the INCRELEX® injection, especially at the beginning of treatment. Your child should always have a source of sugar such as orange juice, glucose gel, candy, or milk available in case symptoms of low blood sugar happen. For severe low blood sugar, if your child is not responsive and cannot drink sugar-containing fluids, you should get emergency medical help for your child right away.
Allergic reactions.
are a serious but common side effect of INCRELEX®. Call your child’s doctor right away if your child gets a rash or hives, which generally appear minutes to hours after the injection and may sometimes occur at many places on the skin. Stop taking INCRELEX® and get medical help right away if your child has trouble breathing or goes into shock, with symptoms like dizziness, pale, clammy skin or passing out. INCRELEX® can also cause reactions at the injection site including: loss of fat (lipoatrophy); increase of fat (lipohypertrophy); pain; redness; or bruising.
Increased pressure in the brain (intracranial hypertension).
INCRELEX®, like growth hormone, can sometimes cause a temporary increase in pressure within the brain. Symptoms include persistent headache and nausea with vomiting.Enlarged tonsils.
Signs include: snoring, difficulty breathing, sleep apnea (a condition where breathing stops briefly during sleep), or fluid in the middle-ear.A bone problem called slipped capital femoral epiphysis.
When the top of the upper leg bone (femur) slips apart. This may lead to a serious condition where bone tissue dies due to a lack of blood supply (osteonecrosis). Get medical help for your child right away if your child develops a limp or has hip or knee pain.Worsened scoliosis.
(caused by rapid growth).Tumor growths.
Several cases of cancerous tumors have been observed in children who received INCRELEX®. Tell your doctor immediately if your child develops any new growths or symptoms of cancer.Benzyl alcohol toxicity.
Benzyl alcohol, a preservative in INCRELEX®, can cause serious side effects, including death in infants.
The most common side effects of INCRELEX® include: Low blood sugar (hypoglycemia); injection site reactions; allergic reactions; and enlarged tonsils.
These are not all the side effects of INCRELEX®. Call your child’s doctor if your child has side effects that are bothersome or that do not go away. You may report side effects to FDA at 1-800-FDA-1088.
How Should My Child Receive INCRELEX®?
The dose of INCRELEX® to be given per injection can vary for each child. Your child’s doctor will determine the appropriate dose by taking into account your child’s weight, height, age, and other factors, such as tolerability. This information will be given to you, along with exact instructions and a dosing sheet.
A summary of how your child should receive INCRELEX® is given here. Detailed INCRELEX® injection instructions can be found in the Patient Information Leaflet and Instructions for Use.

Tips for Injecting INCRELEX®
What You Should Know About INCRELEX® Dosing
Your child’s doctor will determine the correct dose of INCRELEX®. It is important that your child is weighed and measured regularly so their dose can be adjusted by their physician as appropriate. The starting dose of INCRELEX should be between 0.04 and 0.08 mg/kg. If a dose is well-tolerated for at least one week, then the doctor may choose to increase the dose by 0.04 mg/kg per dose, to the maximum dose of 0.12 mg/kg given twice daily. The dose should never be increased to make up for one or more skipped doses. INCRELEX® should not be administered when the meal or snack is skipped.
Because INCRELEX® has insulin-like hypoglycemic effects it should be administered shortly before or after (± 20 minutes) a meal or snack.
Children may show better growth results when their INCRELEX® dose is adjusted by their physician over the course of treatment. Regular weight monitoring is critical for proper dosing.
It is important that your child be weighed and measured regularly (e.g., monthly), so their dose can be adjusted as appropriate. You can keep your own records of your child’s growth, but it may be best to check in with the doctor’s office so you get the most accurate measurements.
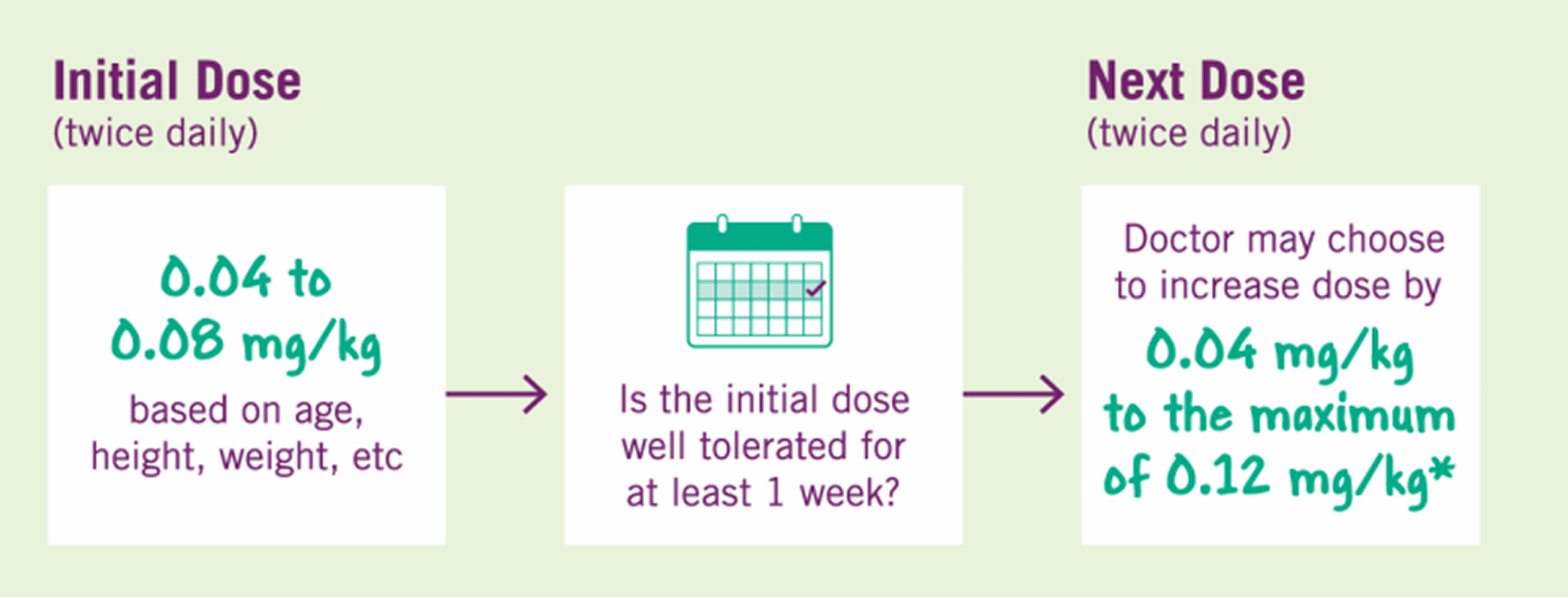
INCRELEX® dosing for Severe Primary IGF-1 Deficiency Treatment*
INCRELEX® should be given 20 minutes before or after eating a snack or meal to reduce the chance of hypoglycemia. If your child is unable to eat shortly before or after a dose for any reason, that dose of INCRELEX® should not be given. If your child misses a dose, do not double a dose to make up for it.
Indication and important safety information
Important safety information
Always give Increlex exactly as your doctor directed.
Do not take INCRELEX if you are allergic to IGF-1 or any of its other ingredients. Report any allergic reactions.
INCRELEX should be injected under the skin and into the muscle. It should not be injected directly into a blood vessel.
INCRELEX should not be used after growth plates close which happens during puberty.
INCRELEX should not be used in children with cancerous tumors or a history of cancer.
- Hypoglycemia (low blood sugar): INCRELEX should be administered 20 minutes before or after a meal or snack and should not be administered when the meal or snack is skipped. Checking blood glucose levels is recommended. The dose of INCRELEX may need to be adjusted until an appropriate dose is decided by your doctor.
- Intracranial Hypertension: Increased pressure in your skull may occur because of cerebrospinal fluid buildup around your brain. Therefore, your doctor may require an eye examination at the start of Increlex treatment and periodically during the time you are taking INCRELEX.
- Lymphoid Tissue Hypertrophy: Lymphoid tissue hypertrophy is a noncancerous increase in the number of immune cells called lymphocytes. Patients should have periodic examinations with your doctor to rule out potential complications.
- Slipped Capital Femoral Epiphysis: Slipped capital femoral epiphysis is a bone problem where the top of the upper leg (femur) slips apart. This may lead to a serious condition where bone tissue dies due to a lack of blood supply (osteonecrosis). Get medical help for your child right away if your child develops a limp or has hip or knee pain.
- Progression of Scoliosis: Your doctor will monitor you during treatment with INCRELEX if you have a history of scoliosis.
- Malignant Neoplasia: There have been reports of cancerous tumors in children who received INCRELEX. It is unknown whether there is any relationship between INCRELEX therapy and new occurrence of tumors. Tumors were mostly reported in patients with rare genetic conditions of short stature associated with a higher risk of cancer, or in patients already at risk of cancer. The tumors were seen more frequently in patients who received INCRELEX at higher than recommended doses or at doses that produced IGF-1 levels above normal for age and sex. Your doctor will carefully monitor you during your treatment with INCRELEX for development of tumors. If cancerous tumors develop, your doctor will stop your INCRELEX treatment.
- Risk of Serious Adverse Reactions in Infants due to Benzyl Alcohol Preserved Solution: Serious and fatal adverse reactions can occur in neonates and infants treated with benzyl alcohol-preserved drugs. Use of INCRELEX in infants is not recommended.
The most common adverse reactions include low blood sugar, reactions at the injection site or throughout your body, and enlarged tonsils.
You are encouraged to report negative side effects of prescription drugs by contacting Eton Pharmaceuticals, Inc. at 1-855-224-0233 or the U.S. Food and Drug Administration (FDA) at www.fda.gov/safety/Medwatch or call 1-800-FDA-1088.
Please see full Prescribing Information for more information.
Indication and important safety information
Important safety information
Always give Increlex exactly as your doctor directed.
Do not take INCRELEX if you are allergic to IGF-1 or any of its other ingredients. Report any allergic reactions.
INCRELEX should be injected under the skin and into the muscle. It should not be injected directly into a blood vessel.
INCRELEX should not be used after growth plates close which happens during puberty.
INCRELEX should not be used in children with cancerous tumors or a history of cancer.
- Hypoglycemia (low blood sugar): INCRELEX should be administered 20 minutes before or after a meal or snack and should not be administered when the meal or snack is skipped. Checking blood glucose levels is recommended. The dose of INCRELEX may need to be adjusted until an appropriate dose is decided by your doctor.
- Intracranial Hypertension: Increased pressure in your skull may occur because of cerebrospinal fluid buildup around your brain. Therefore, your doctor may require an eye examination at the start of Increlex treatment and periodically during the time you are taking INCRELEX.
- Lymphoid Tissue Hypertrophy: Lymphoid tissue hypertrophy is a noncancerous increase in the number of immune cells called lymphocytes. Patients should have periodic examinations with your doctor to rule out potential complications.
- Slipped Capital Femoral Epiphysis: Slipped capital femoral epiphysis is a bone problem where the top of the upper leg (femur) slips apart. This may lead to a serious condition where bone tissue dies due to a lack of blood supply (osteonecrosis). Get medical help for your child right away if your child develops a limp or has hip or knee pain.
- Progression of Scoliosis: Your doctor will monitor you during treatment with INCRELEX if you have a history of scoliosis.
- Malignant Neoplasia: There have been reports of cancerous tumors in children who received INCRELEX. It is unknown whether there is any relationship between INCRELEX therapy and new occurrence of tumors. Tumors were mostly reported in patients with rare genetic conditions of short stature associated with a higher risk of cancer, or in patients already at risk of cancer. The tumors were seen more frequently in patients who received INCRELEX at higher than recommended doses or at doses that produced IGF-1 levels above normal for age and sex. Your doctor will carefully monitor you during your treatment with INCRELEX for development of tumors. If cancerous tumors develop, your doctor will stop your INCRELEX treatment.
- Risk of Serious Adverse Reactions in Infants due to Benzyl Alcohol Preserved Solution: Serious and fatal adverse reactions can occur in neonates and infants treated with benzyl alcohol-preserved drugs. Use of INCRELEX in infants is not recommended.
The most common adverse reactions include low blood sugar, reactions at the injection site or throughout your body, and enlarged tonsils.
You are encouraged to report negative side effects of prescription drugs by contacting Eton Pharmaceuticals, Inc. at 1-855-224-0233 or the U.S. Food and Drug Administration (FDA) at www.fda.gov/safety/Medwatch or call 1-800-FDA-1088.
Please see full Prescribing Information for more information.
