Mechanism of Action
Mechanism of Action
INCRELEX® Mechanism of Action
INCRELEX® contains human insulin-like growth factor-1 produced by recombinant DNA technology (rhIGF-1). IGF-1 is a key hormonal mediator on growth stature.1
Under normal circumstances, GH binds to its receptor in the liver, and other tissues, and stimulates the synthesis and secretion of IGF-1. In target tissues, the type 1 IGF-1 receptor, which is homologous to the insulin receptor, is activated by IGF-1, leading to intracellular signaling which stimulates multiple processes resulting in growth stature.1
The metabolic actions of IGF-1 are in part directed at stimulating the uptake of glucose, fatty acids, and amino acids so that metabolism supports growing tissues.1
Safety Profile
Safety Profile
Established Safety Profile With Increlex
Adverse reactions occurring with Increlex in ≥5% of patients in clinical studies included*1:
- Hypoglycemia, lipohypertrophy, bruising, otitis media, serous otitis media, snoring, tonsillar hypertrophy, headache, dizziness, convulsions, vomiting, hypoacusis, fluid in middle ear, ear pain, abnormal tympanometry, cardiac murmur, arthralgia, pain in extremity, thymus hypertrophy, ear tube insertion
Hypoglycemia was reported by 30 patients (42%) at least once during their course of therapy1
- Most cases of hypoglycemia were mild or moderate in severity
- Five subjects had severe hypoglycemia (requiring assistance and treatment) on one or more occasions and 4 subjects experienced hypoglycemic seizures/loss of consciousness on one or more occasions
- 14 of the 30 patients (47%) reporting hypoglycemia had a history of hypoglycemia prior to treatment
- The frequency of hypoglycemia was highest in the first month of treatment and episodes were more frequent in younger children
- Symptomatic hypoglycemia was generally avoided when a meal or snack was consumed either shortly (i.e., 20 minutes) before or after the administration of INCRELEX®
No patients withdrew from any clinical study because of adverse reactions1
*In clinical studies of 71 patients with Primary IGFD treated for a mean duration of 3.9 years and representing 274 patient years.2
Growth rates
Growth rates
Improve Growth Rates in Children With Severe Primary IGF-1 Deficiency
In clinical trials, INCRELEX® improved statural growth in patients diagnosed with SPIGFD.1
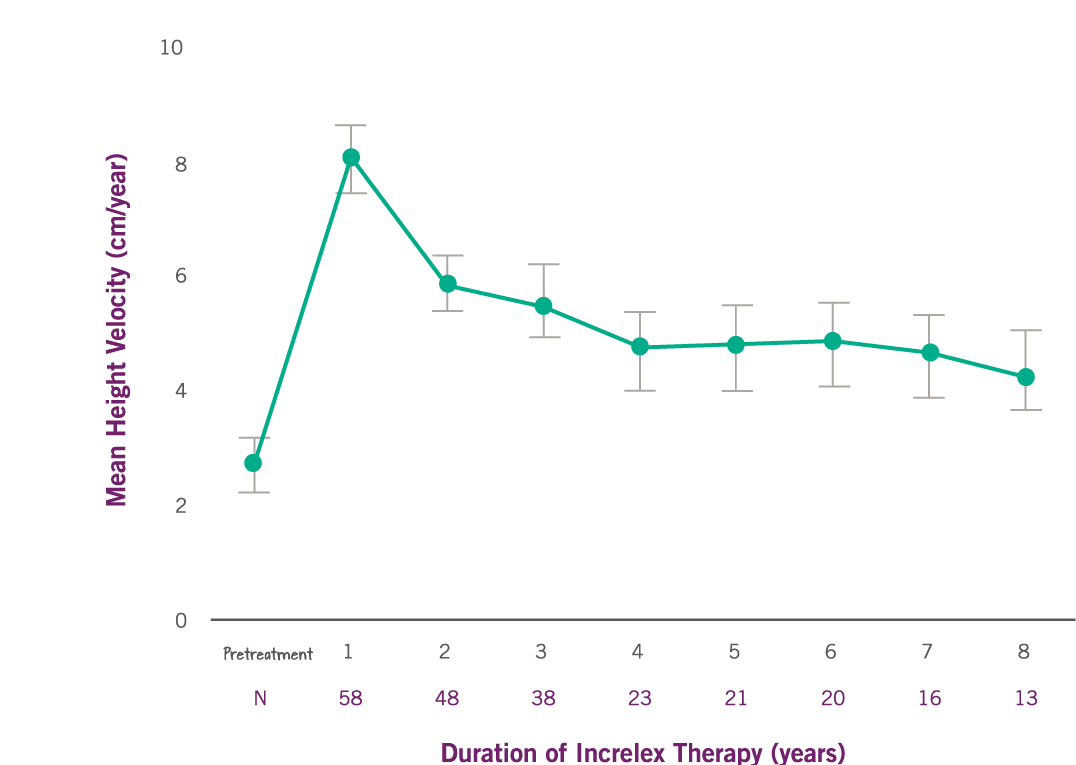
Growth Rate with INCRELEX® over 8 years1
Data (95% confidence intervals) shown are from individuals treated continually in five pooled clinical studies (four open-label and one double-blind, placebo-controlled) conducted in 71 pediatric subjects with Severe Primary IGF-1 Deficiency. Pretreatment height velocity was available for 58 subjects. Adapted from INCRELEX® full prescribing information.1
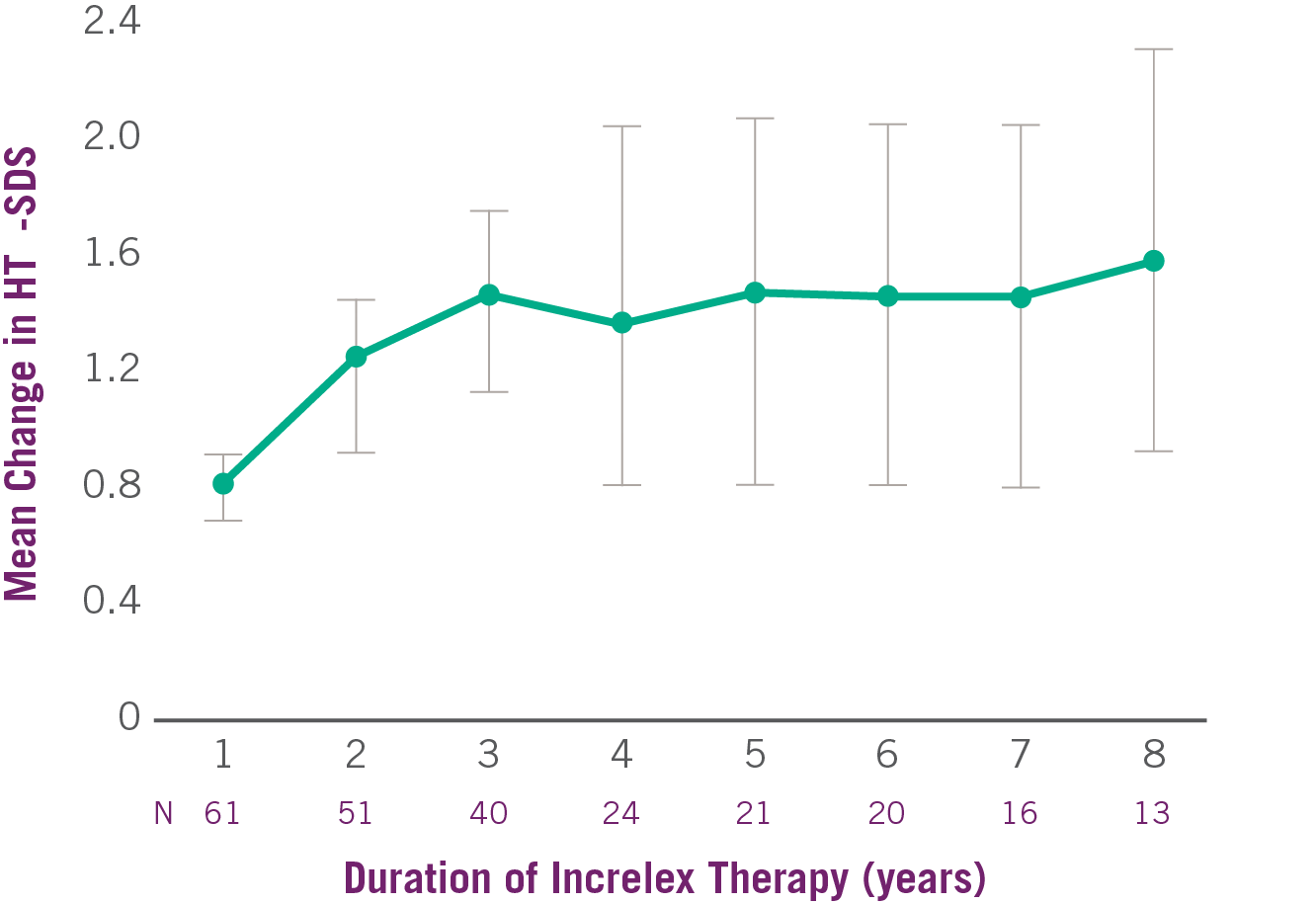
- Average height SDS had increased from start of treatment by +0.8 at Year 1 and +1.2 at Year 2.1
- The average height SDS was -6.7 at baseline for the 61 subjects in the efficacy analysis.
- Mean height velocity nearly tripled over baseline in the first year (P<0.0001).1
- Mean height velocity increased to 8 cm/year in the first year, on average, from a baseline of 2.8 cm/year (P<0.0001).
- Mean height velocity was sustained at approximately 5 cm/year in Years 2 through 6 of treatment.
- Change in bone age progressed in accord with advancing chronological age.1
- Forty-nine subjects were included in an analysis of the effects of INCRELEX® on bone age advancement.
- The mean ± standard deviation (SD) change in chronological age was 4.9 ± 3.4 years.
- The mean ± SD change in bone age was 5.3 ± 3.4 years.
Change in Height SDS with INCRELEX® Over 8 Years1
Adapted from INCRELEX® full prescribing information.1 SDS, standard deviation score.1 Data (95% confidence intervals) shown are from individuals treated in five pooled clinical studies (four open-label and one double-blind, placebo-controlled) conducted in 71 pediatric subjects with severe primary IGFD. The average height SDS was -6.7 + or – 1.8 at baseline for the 61 subjects in the efficacy analysis.
Dosing & Administration
Dosing & Administration
INCRELEX® Dosing and Administration
Increlex Dosing & Injection video
The dosing of INCRELEX® should be individualized for each patient. This should be based on the periodic assessment of each patient’s weight, tolerability, and laboratory parameters.1 Patients should start INCRELEX® treatment on a weight-based dose in the recommended starting dose range of 0.04 to 0.08 mg/kg twice daily, given subcutaneously.1
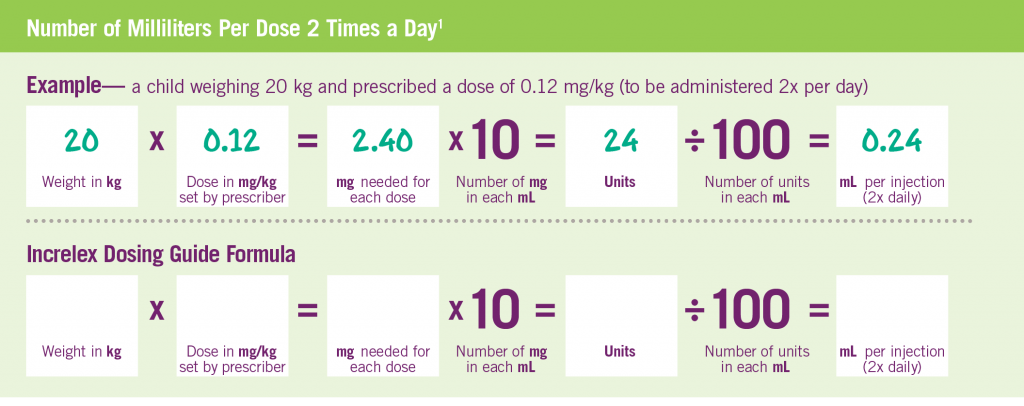
INCRELEX® Dosing is Weight-based1
Adapted from INCRELEX® full prescribing information. The following formula was used to calculate the dosage as units per injection: patient body weight (kg) x single dose of Increlex (mg/kg) x 1 mL/10 mg x 100 units/1 mL† = units/injection. Two doses per day, morning and evening before or after (plus/minus 20 minutes) a meal or snack (a separation of 12 hours) are recommended.1 †1 mL=1 cc.
Pre-prandial glucose monitoring is recommended at treatment initiation and until a well-tolerated dose is established. If frequent symptoms of hypoglycemia or severe hypoglycemia occur, pre-prandial glucose monitoring should continue. If hypoglycemia occurs with recommended doses despite adequate food intake, the dose should be reduced.1
If the starting dose of INCRELEX® is tolerated for at least 1 week, the dose may be increased by 0.04 mg/kg per dose up to the maximum dose of 0.12 mg/kg given twice daily.1 Doses greater than 0.12 mg/kg given twice daily have not been evaluated in children with primary IGFD and, due to potential hypoglycemic effects, should not be used.1
Appropriate dose adjustments of INCRELEX® are necessary to avoid administering a dose that may be too low for an individual patient. Patients should be weighed and measured frequently, as regular monitoring is critical for proper unit dosing.1
Three Simple Steps to INCRELEX® Dosing1
Adapted from INCRELEX full prescribing information.1 BID, twice daily. *Doses greater than 0.12 mg/kg given BID have not been evaluated in children with primary IGFD and, due to the potential risk of neoplasia and hypoglycaemic effects, should not be used. If hypoglycemia occurs with recommended doses despite adequate food intake, the dose should be reduced.1 Pre-prandial glucose monitoring is recommended at treatment initiation and until a well-tolerated dose is established.1 If frequent symptoms of hypoglycemia or severe hypoglycemia occur, pre-prandial glucose monitoring should continue.1
INCRELEX® Administration1
See Instructions for Use in Package Insert for Full Details on How to Administer Increlex.1
Supply
Supply
INCRELEX® Supply
INCRELEX® is supplied in a multiple dose glass vial at a concentration of 10 mg per mL (40 mg per vial).1
When prescribing a 30-day supply of INCRELEX® your patients will need a specific number of vials depending on their body weight and dosage regimen as illustrated below.1,3
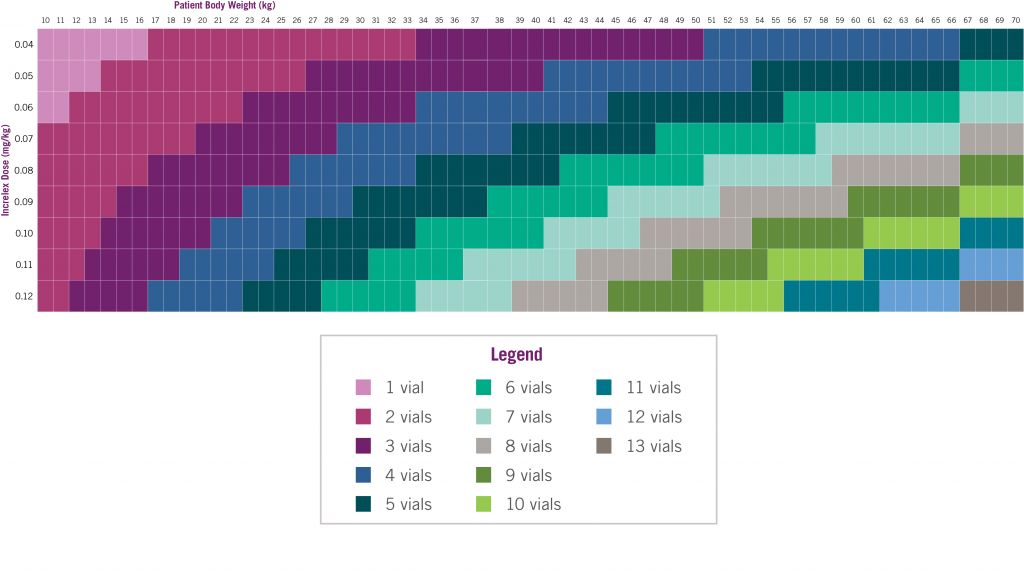
Number of INCRELEX® vials to prescribe for a 30-day supply*4
The following formula was used to determine the number of vials per month: patient body weight [kg] x single dose of Increlex [mg/kg] x 2 doses per day x 30 days per month/40 [mg/vial] = vials/month.4 *If using syringes that measure dose in units, doses in mg/kg must be converted to units using the following formula: patient body weight [kg] x single dose of Increlex [mg/kg] x 1 mL/10 mg x 100 units/1 mL = units/injection.1